About
*Now offering 31.5 CEs!
USP General Chapter <800>, which became official December 1, 2019, offers guidelines for the safe handling of hazardous drugs. USP <800> also stipulates that each entity handling Hazardous Drugs (HDs) MUST identify a Designated Person (DP) to guide them through initial implementation and to ensure ongoing compliance.
Our HDDP Certification course is a self-paced, cost-effective way to train the employee who is to assume the required role of designated person, certifying that he/she has the knowledge to ensure your compliance with USP <800>.
The self-paced study guide is divided into two sections – Administrative Topics and Handling Hazardous Drugs – with a total of 14 different modules. Each module covers a topic that the DP is required to be familiar with. These topics include:
- Part 1: Administrative Topics
- Module 1: Background and Program Management
- Module 2: Developing and Maintaining a List of Hazardous Drugs
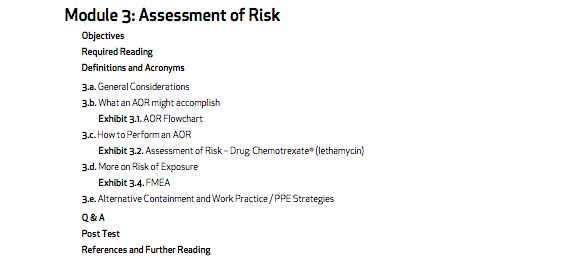
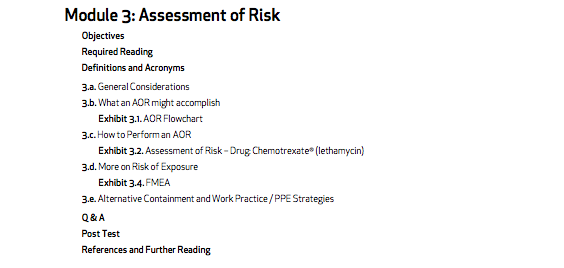
- Module 3: Assessment of Risk
- Module 4: References and Resources, Standard Operating Procedures, Training, and Competency
- Module 5: Facilities andEquipment
- Module 6: Personal ProtectiveEquipment
- Part 2: Handling Hazardous Drugs
- Module 7: Receipt and Storage
- Module 8: Compounding
- Module 9: Labeling, Packaging, Dispensing, Transport, and Administration
- Module 10: Deactivation, Cleaning, and Disinfection
- Module 11: Disposal
- Module 12: Spills and Exposure Events
- Module 13: Environmental Monitoring and Medical Surveillance
- Module 14: Reaching and Maintaining USP <800> Compliance
Below you will find examples of Modules 1, 3, 7, 11 and 13.
The Process to become HDDP Certified
The Hazardous Drugs Designated Person certification is designed to be a self-paced and cost-effective solution for compliance with the USP <800> requirement for a designated person.
After purchase:
- You will be sent a downloadable comprehensive study guide that will prepare you for an exam
- You will be given a specific login for the exam that will be emailed to you
- We suggest spending at least 40 hours going over the course information to be prepared for the exam
- You will be given two chances to pass the exam
- Once you pass the exam, you are certified as an HDDP and can act as the required DP for your organization handling Hazardous Drugs
What is the Hazardous Drug Designated Person responsible for?
- Creating and implementing procedures concerning HDs
- Performing a documented annual review of these Standard Operating Procedures (SOPs)
- Monitoring compliance with these SOPs and relevant rules and regulations
- Ensuring worker training and competency for HDs
- Ensuring "environmental control" of areas where HDs are found and handled
- Overseeing facility monitoring, managing related documents, and acting on results, including incident reports concerning HDs
Who should become an HDDP?
Anyone handling Hazardous Drugs for a healthcare organization can become a Hazardous Drug Designated Person.
Handling means being involved in any part of the process, from compounding the drug, administering the drug to the patient, and/or disposing of the drug. An organization can identify one employee or have multiple employees certified to ensure compliance and safety. Anyone from the pharmacy technician in a compounding pharmacy to a nurse handling hazardous drugs in a home health company may need to become the HDDP for their healthcare facility.
If you have questions about whether you should consider being the Designated Person for your organization, reach out to us!
What does USP <800> say about a Designated Person?
*Image of the actual USP <800> document from: http://www.usp.org/sites/default/files/usp/document/our-work/healthcare-quality-safety/general-chapter-800.pdf page 3